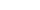Baysient announces updates to its therapeutic drug monitoring app Time To Target (T3) to enable clinicians to deliver personalized dosing recommendations for the subcutaneous forms of Infliximab and adalimumab.
Inflammatory bowel disease (IBD) is a chronic condition affecting approximately 3 million US adults. One of the most effective and commonly prescribed biologic treatments for IBD is Infliximab, which can be administered either intravenously or subcutaneously (with the current subcutaneous formulation available outside the U.S. called Remsima™ SC). Another IBD treatment option, adalimumab (currently approved under the brand names Humira™, Idacio®, Yusimry™, Hulio™, Abrilanda™, Hadlima™, Hyrimoz®, and Cyltezo®), is only available for subcutaneous administration.
Traditional Methods Risk Drug Failure
While Infliximab and adalimumab are both effective treatment options, a proportion of patients lose response to these therapies within the first year, partly due to the formation of anti-drug antibodies (ADA). Other patients fail these therapies because their exposure to the drug is too low to be effective, as labeled dosing of monoclonal antibodies (mAbs) does not consider the variation in patient clearance.
Using TDM to Increase Durability of mAbs
Therapeutic drug monitoring (TDM) has been shown effective in increasing the durability and reducing the immunogenicity of intravenous Infliximab, allowing patients in maintenance to attain and sustain a therapeutic drug trough level, improving their long-term drug response. To address this, Baysient has developed two therapeutic drug monitoring protocols based on mAb pharmacokinetics to be used with the subcutaneous forms of these drugs.
TDM with T3
One approach – Time To Target® (T3) – is a web-based, individualized patient nomogram/algorithm specifically developed to easily determine precision dosing in maintenance based on real patients’ clinical characteristics (weight, dose, dose interval and target trough) and a measured trough concentration. T3’s approach takes into account clearance differences amongst patients, allowing healthcare professionals to adjust dosages of subcutaneous Infliximab and adalimumab accordingly.
TDM with iDose
The second approach – iDose® – is similar to T3, except iDose may be used for both Infliximab induction and maintenance and can be updated without additional measured concentrations. T3 is for maintenance only and requires a measured concentration, but it can determine the individual half-life of a drug for each patient. By having this information, an accurate indication of the length of time until the drug concentrations fall below the target concentration can be determined.
Subcutaneous Administration Can Be Effective and Convenient But Risks Drug Failure or Adverse Events Due to Standard Dosing Regimens
The subcutaneous treatment option is cost-effective as it replaces intravenous infusion encounters with self-administration.
However, although subcutaneous administration of Infliximab or adalimumab is convenient, reliance on standard dosing regimens means patients often have a much higher or lower exposure than needed. The labeled dosing is for the ‘typical patient’ and does not consider the various factors impacting how an individual’s body responds and processes these therapies.
Without TDM, patients are often either unable to tolerate the amount of drug in their bodies or unable to respond to it. They also risk reaching drug failure or experiencing adverse events due to supratherapeutic or subtherapeutic concentrations.
If you have ever tried using TDM with adalimumab and been disappointed that the patient’s concentration did not increase to your target within a new dose or two, there is a reason for this, and T3 can help you see what is happening. Because of the relatively low dose of adalimumab in each pen, when administered to patients with shorter half-lives, it takes more than one dose to get a patient to a new target trough because the drug accumulation over several doses is necessary to achieve the new steady state desired target.
Figure 1
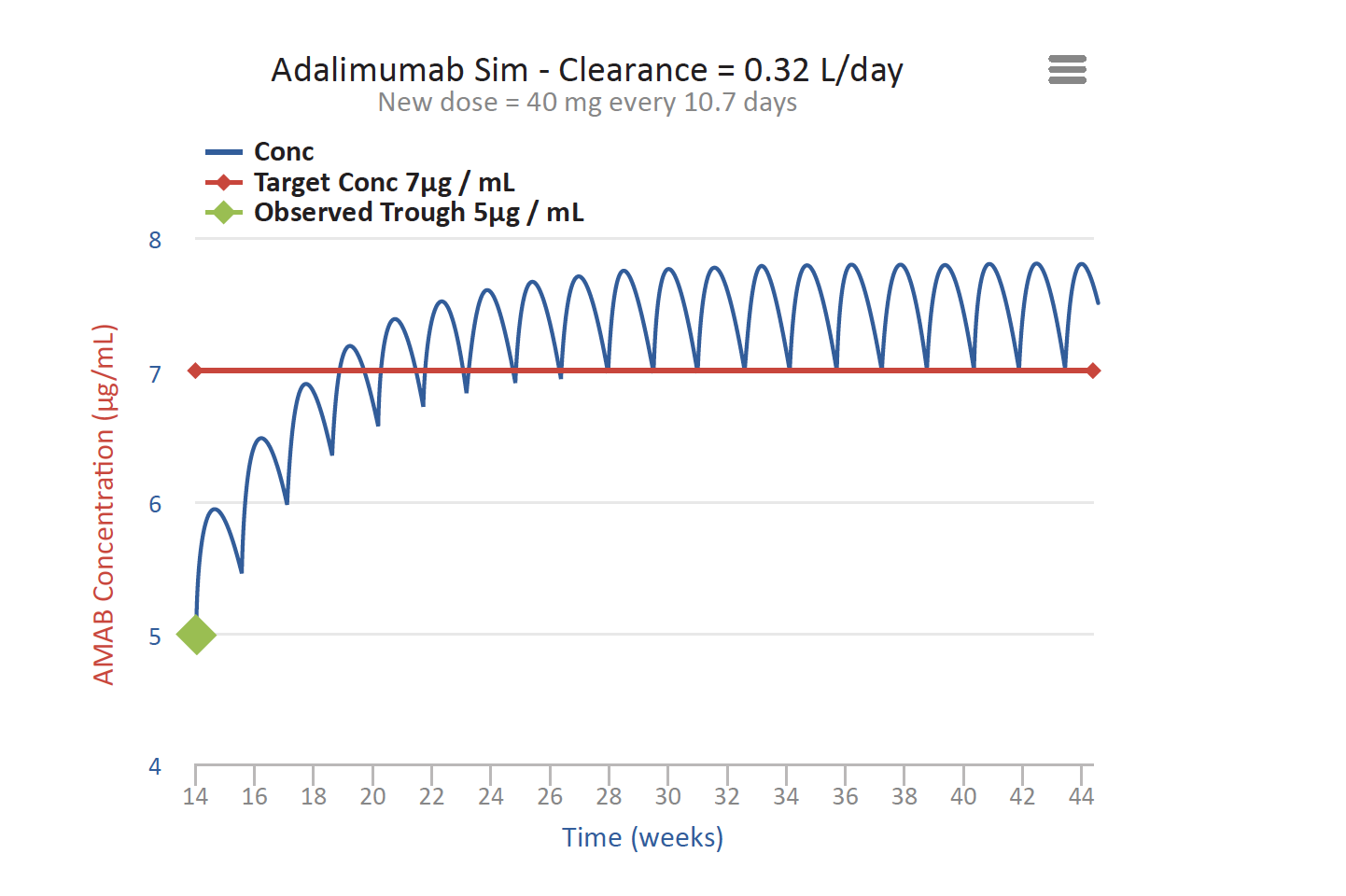
For patients getting subcutaneous Infliximab, the problem is often quite different because the amount of drug in the Infliximab pen is much greater than the amount in the adalimumab pen described above. Patients treated with subcutaneous Infliximab tend to have very high trough concentrations. In fact, the reported mean high trough concentrations from subcutaneous Infliximab is 20 ug/mL, twice what is usually desirable. High exposure to Infliximab can result in adverse events such as myalgia, body aches and/or pain. Furthermore, Infliximab (along with other monoclonal antibodies used to treat IBD) are immunosuppressants and carry black box warnings. Therefore, a treating physician may wish to change the dose to lower the trough to alleviate these events or reduce the likelihood of infections, etc. T3 can help with this process as well.
Figure 2
If a patient has a low trough level with subcutaneous Infliximab, then T3 can help adjust the dose to improve this problem.
Therapeutic Drug Monitoring with T3: The Key to Maintaining Drug Efficacy
TDM involves measuring specific drugs or their breakdown products at regular intervals to keep the concentration of medication in the blood relatively consistent. This process is essential for enhancing drug efficacy and reducing toxicity.
T3 determines the amount of drug in the patient’s body, the biological half-life of the drug, and calculates the time it will take for the concentration of the drug to reach the desired target level determined by the physician. This helps clinicians understand when that patient needs to receive another subcutaneous dose.
T3 Prioritizes Timing of the Dose, Not the Dose Itself
Individual patient half-life is not considered when following the standard subcutaneous Infliximab and adalimumab dosing labels. Instead, patients receive a set dose on a set schedule, despite the fact individual patient characteristics and responses to the drug can vary greatly.
While some physicians may prescribe more of a drug, increasing the dose doesn’t always fix the problem in patients with a short half-life. Low troughs can remain a problem because the dose does not alter a drug’s half-life. Increasing the dose may temporarily maintain adequate concentration early in the dosing interval, but the extra drug does little to extend later-term drug concentration.
In order for patients to maintain sufficient drug concentration during subcutaneous treatment with Infliximab and adalimumab, clinicians must take into account the patient’s individualized factors for regulating drug levels.
T3 Helps Patients Reach Steady-state Drug Concentrations
T3 enables clinicians to easily personalize the dosing regimen of subcutaneous Infliximab and adalimumab based on the timing of individual half-life, ensuring the drug remains at an effective concentration in the body.
For a patient treated with adalimumab, it can take roughly five doses for their body to reach and maintain a new therapeutic target concentration of the drug in their system (see Figure 1 above).
Similarly, patients treated with subcutaneous Infliximab may require several doses to bring their drug concentration down to a steady state (see Figure 2 above). T3 helps clinicians identify when to administer the next dose and, in turn, achieve and maintain an effective concentration of the drug.
T3 Improves Long-term Drug Response of Subcutaneous Infliximab and Adalimumab
With subcutaneous Infliximab and adalimumab offering patients the convenience of at-home administration and reduced healthcare costs associated with fewer clinic visits, maintaining long-term drug response is crucial. Not only does the patient population demand this convenient option, but maintaining drug response helps ensure healthcare costs associated with treatment remain low.
Individualized dosing regimens through T3 are essential for improving long-term drug response, lowering the incidence of anti-drug antibodies and reducing treatment failure, or in some cases reducing the ill effects of too much drug in circulation. Clinicians can use the T3 system to ensure their patients receive the right amount of Infliximab and adalimumab during subcutaneous therapy to maintain a clinically effective drug trough level.
Schedule a T3 Demo Today
T3 is a comprehensive solution for tracking, monitoring and adjusting dosing regimens for subcutaneous Infliximab and adalimumab therapy. To learn more about how T3 can help you maintain the efficacy of subcutaneous mABs in your practice, schedule a demo today.
Our team will explain the features and benefits of T3 and how you can get started with this easy-to-use and intuitive app.